Vaccine Storage Packaging Market Size, Share, Trends and Growth Forecast
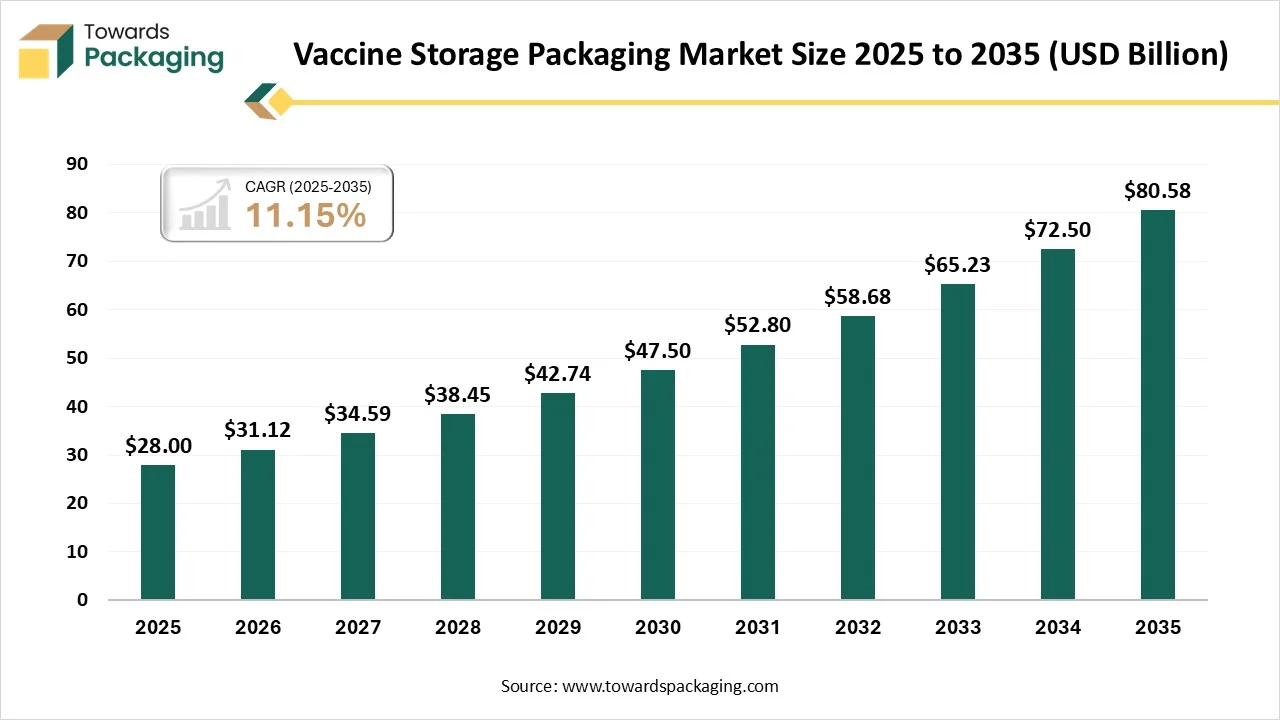
The vaccine storage packaging market is forecasted to expand from USD 31.12 billion in 2026 to USD 80.58 billion by 2035, growing at a CAGR of 11.15% from 2026 to 2035. The demand for vaccine storage and packaging is developing due to the demand to track strict cold chain integrity, ensure the capability of worldwide distribution, and protect against spoilage, too.
Major Key Insights of the Vaccine Storage Packaging Market
- In terms of revenue, the market is valued at USD 28 billion in 2025.
- The market is projected to reach USD 80.58 billion by 2035.
- Rapid growth at a CAGR of 11.15% will be observed in the period between 2026 and 2035.
- By region, North America dominated the global market by holding the highest market share in 2025.
- By region, Asia Pacific is expected to grow at the fastest CAGR from 2026 to 2035.
- By storage equipment type, the refrigerators segment dominated the market in 2025.
- By storage equipment type, the ultra-low temperature freezers segment will be growing at a significant CAGR between 2026 and 2035.
- By packaging type, vials dominated the market in 2025.
- By packaging type, the prefilled syringes segment will be growing at a main CAGR between 2026 and 2035.
- By vaccine type, the mRNA vaccine segment dominated the market in 2025.
- By vaccine type, the DNA vaccines segment will be developing at a main CAGR between 2026 and 2035.
- By application, the human vaccines segment dominated the market in 2025.
- By application, the animal vaccines segment will be growing at a significant CAGR between 2026 and 2035.
- By end-use, the hospitals and clinics segment dominated the market in 2025.
- By end-use, research institutions segment will be developing at a main CAGR between 2026 and 2035.
What is Vaccine Storage Packaging?
Vaccines are among the most complicated machines in current public health, which prevent millions from infectious diseases each year. Their smoothness, hence, does not completely give feedback on the science behind their growth. After packaging, vaccines are kept in temperature-controlled facilities till they are ready for shipment. A high-level temperature monitoring system constantly manages conditions that alert staff to some changes from the prescribed series.
Vaccine Storage Packaging Market Trends
- Expansion of cold chain infrastructure: Growing use of temperature-sensitive vaccines has increased demand for reliable cold and ultra-cold storage solutions. Advanced insulated packaging ensures vaccines remain effective during storage and long-distance transportation.
- Adoption of smart packaging: Integration of IoT sensors and data loggers enables real-time temperature monitoring, improving traceability, reducing spoilage, and ensuring compliance with regulatory standards.
- Shift toward sustainable packaging: Manufacturers are adopting recyclable and reusable materials to reduce environmental impact while maintaining safety, performance, and regulatory compliance in vaccine distribution.
Technological Developments in the Vaccine Storage Packaging Market
Emerging technologies are updating vaccine storage and distribution, which serve inventive solutions to solve the challenges witnessed by the pharmaceutical sector. Cold chain monitoring systems use IoT (Internet of Things) technology to manage vaccine temperature and other surrounding conditions in real-time. Such systems use wireless connectivity and sensors to constantly track and alert healthcare servers or the logistics personnel in terms of temperature excursions. Such technology allows proactive mediations to protect the vaccine from spoilage and ensure the integrity of the cold chain. On the other hand, the COVID-19 pandemic has increased the use of drones for the worldwide delivery of vaccines and medicines. Such development has been proven highly beneficial in spaces that experience limited access and geographical barriers to refrigerated transportation, in which challenges are specifically marked.
Trade Analysis of Vaccine Storage Packaging Market: Import & Export Statistics
- Between July 2024 and June 2025, as buyers globally imported 366 shipments of vaccine packaging.
- Such shipments were being assisted by 366 exporters and bought by 264 global buyers.
- Ukraine, Colombia and Vietnam have come up as the three leading importing countries.
- On the other hand, the United States, Argentina, and Germany are the leading exporting countries.
- The United States exported 1,389 shipments, Argentina exported 742 shipments, and Germany exported 517 shipments, which ranks as the biggest worldwide vaccine packaging exporters.
Vaccine Storage Packaging Market - Value Chain Analysis
- Package Design and Prototyping: The cold chain is complicated for the smooth and safe delivery of vaccines globally. To make a successful cold chain, careful planning, an effective system of balance and checks, and attention to detail are important. With more organizations that request development availability to vaccinations globally, companies must ensure they have relevant cold chain materials.
- Recycling and Waste Management: Dry ice should be avoided from transporting containers before they are kept in cold rooms or freezer rooms. It must be enabled to redirect back into gas in a perfectly ventilated space at the correct room temperature and not left behind in an unprotected area, disposed of via vial drains or flush toilets, or in the trash.
- Logistics and Distribution: Vaccines are transported and stored in temperature-controlled cold chain packaging to maintain their safety and potency. Big hospitals and companies make sure that under strong cold chain conditions between (2 degrees Celsius - 8 degrees Celsius) by using an insulated container or polybags with gel packs.
Segmental Insights
Storage Equipment Type Insights
How the Refrigerators Segment Dominated the Vaccine Storage Packaging Market in 2025?
The refrigerators segment dominated the vaccine storage packaging market in 2025, as healthcare servers are needed for vaccine storage at the public health units. It is suggested that aim-constructed refrigerators are used for storage, which are suitable for large inventories of vaccines. Although it is a purpose-built refrigerator that is currently more costly than the domestic (kitchen) and bar refrigerators, it has the benefit that updates are not needed for vaccine storage. The crucial characteristics of purpose-built refrigerators include temperature regulation, which ensures that the interior temperature is maintained.
The ultra-low temperature freezers segment is projected to experience the fastest CAGR during the forecast period. Ultra-low temperatures are achieved through a path that refrigerants can take with the help of rigid compressors. As two of the different and independent refrigerants pass through classified compressor chambers, they pass through high and low temperature refrigeration loops. As they travel, they experience a range of thermodynamic procedures which assist in making the Arctic surroundings in the ULT chamber.
Packaging Type Insights
How Vials Segment Dominated the Vaccine Storage Packaging Market in 2025?
The vials segment dominated the market in 2025 because the latest vial packaging machines mix robotics, sensors, and advanced control machines that are constantly tracked as variables such as pressure, weight, and temperature. Such systems use real-time response loops to adjust on the fly, to ensure minimal wastage and continuation. Several machines also include modular patterns that can be updated or accepted as the product lines get updated, which serves long-term flexibility in the manufacturing environment.
The prefilled syringes segment is expected to witness the fastest CAGR during the forecast period. They deliver various advantages, which include developed drug partition effectiveness, lower dosage error, reduced waste, and convenient administration. Hence, terminal sterilization of prefilled syringes is an urgent procedure that serves several issues for the pharmaceutical producers, which must be solved. They are frequently produced in a container of 100 units. This assists in allowing high-capacity carrying for the injectable products. Additionally, the sterilization of prefilled syringes needs accurate control over pressure, temperature, and assistance to ensure product integrity and constant sterility results.
Vaccine Type Insights
Why the mRNA Segment Dominated Vaccine Storage Packaging Market in 2025?
The mRNA segment dominated the vaccine storage packaging market in 2025 as the only mRNA vaccines that are accepted for human usage are those grown against COVID-19. It is particularly the Comirnaty vaccine offered by Pfizer-BioNTech and the Spikevax by Moderna, which uses mRNA technologies to protect against SARS-CoV-2.Such vaccines have been administered to hundreds of millions of people globally with high effectiveness in preventing severe COVID-19.
The DNA vaccines segment is projected to witness the fastest CAGR during the forecast period. Their packaging contains two different but linked ideas, such as molecular packaging and pharmaceutical packaging, as the vaccine is distributed and stored. Lipid nanoparticles are the same as mRNA vaccines, as DNA can be included in lipid nanoparticles to prevent it from being degraded and provide its penetration across cell membranes. DNA is sometimes packaged inside the protein shell, which is a toxic virus, such as an adenovirus. Such a virus behaves as a delivery truck that naturally knows how to fill DNA into a cell’s nucleus.
Application Insights
Why the Human Vaccines Segment Dominated the Vaccine Storage Packaging Market in 2025?
Human vaccines dominated the vaccine storage packaging market in 2025, as vaccines are fragile medicines that should be protected from temperature and light changes outside of a range of +2 degrees Celsius to +8 degrees Celsius. All the immunization servers are totally responsible for storing, ordering, receiving, and administering the vaccines, and they should understand the rules of cold chain management and vaccine storage. The National Vaccine Storage Guidelines serve as perfect practice principles for carrying vaccines and tracking the cold chain. The NSW Health Vaccine Storage and Cold Chain Management Policy serves the compulsory need for the management and storage of vaccines in the NSW public facilities.
The animal vaccines segment is expected to experience the fastest CAGR during the forecast period. Temperature-sensitive products, such as biologics, vaccines, and insulin, have proteins or active contents that are highly sensitive to changes in temperature. Vaccines such as rabies and other animal vaccines generally need to be kept within a certain temperature range for their effectiveness. Insulin is applicable to diagnose in pets, as insulin must be officially stored at a temperature range between 2 degrees Celsius and 8 degrees Celsius, as it can mainly change its capability, which can lead to serious health risks for the diabetic animals.
End-User Insights
How Hospitals and Clinics Segment Dominated the Vaccine Storage Packaging Market in 2025?
The hospitals and clinics segment dominated the vaccine storage packaging market in 2025, as small healthcare facilities, specifically in low-income countries, where electricity supply is irrelevant. Vaccine carriers serve as a temporary cold storage solution, which ensures vaccines stay effective till they can be utilized. Additionally, vaccines need to be shifted from a crucial storage facility for smaller clinics or temporary immunization facilities.
The research institutions segment is expected to experience the fastest CAGR during the forecast period. For the clinical trials, such vaccines also include tracking and in-depth records of numbers, chain-of-custody documentation, and temperature logs. Precious documentation assists regulatory compliance, allows for smooth clinical trial chain tracking, and assists in managing any irregularities or negative events during such a study. From packaging and production to transport, storage, and the final distribution, coordination and planning are necessary to make sure vaccines stay safe and ready for usage.
Regional Insights
How North America Dominated the Vaccine Storage Packaging Market?
North America dominated the vaccine storage packaging market in 2025 as it is at the forefront of worldwide healthcare innovation, which is driven by the region’s extremely strong health facilities, growing demand for smooth vaccine distribution, and high immunization rates. As the globe continues to face developing infectious diseases and the current COVID-19 pandemic, the priority is to develop relevant packaging and storage solutions that are not complicated.
How is the Vaccine Storage Packaging Market Growing in Canada?
Canada’s Health and Biosciences Economic Strategy table is being classified as a range of targets for Canada's life science industry, which includes doubling Canada's bioscience and health exports and doubling the number of bioscience and health companies in this industry in the current year. It has been predicted that a biologics production initiative can lead to further growth of domestic potential, which can directly give feedback to actions requested by HBEST, including the development of invention acceptance.
Why is the Vaccine Storage Packaging Market Growing Rapidly in the Asia Pacific?
Asia Pacific expects the fastest growth in the market during the forecast period. Asia Pacific vaccine storage packaging is experiencing fast development driven by growing vaccination programs and developing awareness about vaccine storage. Healthcare providers and governments across the region are investing excessively in the cold chain infrastructure to ensure smoothness. Improved healthcare access, fast urbanization, and growing immunization coverage further proposed market demand. Furthermore, technological developments in terms of refrigeration machines, like smart temperature tracking and energy-efficient designs, are encouraging the product request.
Why is India using the Vaccine Storage Packaging Market Importantly?
The Indian pharmaceutical cold chain industry is currently showing direction and timeline of unexpected transformation, which is classified by the same convergence of strong regulatory deadlines, moving market scenes, and fast technological developments too. As the sector moves nearer the financial and calendar achievements, it experiences a decent “Compliance Cliff” that is driven by the updated Schedule M principles and latest energy efficiency compulsions from the Bureau of Energy Efficiency (BEE).
Recent Developments
- In June 2025, Tjopack, which is a worldwide contract packaging company, revealed a growth of its contract packaging and cold chain storage services in both the United States and the Netherlands.
- In June 2025, Boehringer Ingelheim disclosed a single-dose poultry vaccine in India that delivers prevention against avian disease: Newcastle disease, bursal disease, and Marek’s disease.
Top Companies in the Vaccine Storage Packaging Market
- DHL
- Lineage, Inc
- Thermo Fisher Scientific, Inc
- American Biotech Supply
- PHCbi ( PANASONIC HEALTHCARE CO., LTD)
- MCKESSON CORPORATION
- DB SCHENKER
- Amerisource Bergen Corporation
Vaccine Storage Packaging Market Segments Covered
By Storage Equipment Type
- Refrigerators
- Freezers
- Cryogenic Storage Units
- Ultra-Low Temperature Freezers
By Packaging Type
- Vials
- Syringes
- Prefilled Syringes
- Bulk Packaging
By Vaccine Type
- mRNA Vaccines
- DNA Vaccines
- Protein-Based Vaccines
- Viral Vector Vaccines
By Application
- Human Vaccines
- Animal Vaccines
By End-User
- Hospitals and Clinics
- Pharmacies
- Research Institutions
- Government Agencies
By Region
- North America
- U.S.
- Canada
- Mexico
- Rest of North America
- South America
- Brazil
- Argentina
- Rest of South America
- Europe
- Western Europe
- Germany
- Italy
- France
- Netherlands
- Spain
- Portugal
- Belgium
- Ireland
- UK
- Iceland
- Switzerland
- Poland
- Rest of Western Europe
- Eastern Europe
- Austria
- Russia & Belarus
- Türkiye
- Albania
- Rest of Eastern Europe
- Asia Pacific
- China
- Taiwan
- India
- Japan
- Australia and New Zealand
- ASEAN Countries (Singapore, Malaysia)
- South Korea
- Rest of APAC
- MEA
- GCC Countries
- Saudi Arabia
- United Arab Emirates (UAE)
- Qatar
- Kuwait
- Oman
- Bahrain
- South Africa
- Egypt
- Rest of MEA

